Methyl Iodide is a chemical compound that is relatively volatile. It is commonly found in the form of a dense, colorless liquid. It is also known as iodomethane and has the chemical formula CH3I (one carbon atom with three hydrogen atoms and a single iodine atom connected to it). Methyl iodide is a naturally occurring chemical compound that rice plantations emit in small amounts.
Methyl Iodide Properties
Methyl Iodide is a colorless liquid that turns purple when exposed to light for a long period of time because it is an organoiodide. For this reason, methyl iodide is stored in dark colored bottles. It is soluble in organic solvents and has a boiling point of 41-43 degrees Celsius (105-110 degrees Fahrenheit) depending on its purity. Copper or silver wire can stabilize methyl iodide.
If methyl iodide is not stabilized, it can cause explosions and damages that are very difficult to control due to its toxicity.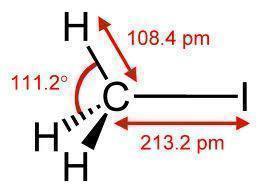
How Methyl Iodide is Created
Methyl iodide can be created in several ways. The reactions that create it often produce byproducts that have various uses. The resulting methyl iodide must be purified by removing any unnecessary chemicals or by distilling it from the compound.
When iodine is added to methanol with red phosphorus, the resulting exothermic reaction provides the appropriate chemical reaction process:
3 CH3OH + PI3 = 3 CH3I + H3PO3
Combining dimethyl sulfate and potassium iodide with calcium carbonate results in the following reaction process:
(CH3O)2SO2 + KI = CH3I + CH3OSO2OK
The resulting methyl iodide in both processes can be purified in a distillation process. The excess iodine can be removed from the solution with a wash of Na2S2O3.
A reaction between methanol and potassium iodide can produce methyl iodide if an acidic catalyst is used. This is illustrated in the following reaction process:
CH3OH + KI + H2SO4 = CH3I + K2SO4 + H2O
The reaction’s low temperature creates irreversible compounds as the sulfuric acid and potassium iodide consume the methanol, creating methyl iodide, potassium sulfate, and water as byproducts. The methyl iodide can then be purified by distilling the mixture.
Chemical Reactions of Methyl Iodide
Since Methyl Iodide is a volatile chemical, it is naturally very reactive and has several different uses. It is also dangerous when handled improperly. With the appropriate handling, Methyl iodide can be safe when used as a reactive compound.
As a methylation reagent, methyl iodide can be used to add methyl groups to substrates such as phenols, amines, thiols, or carboxylic acids. It is a more expensive alternative to chlorides and bromides, which can be used for the same purpose. However, since methyl iodide is a liquid, it can be handled easier than gaseous methylation reagents. Since it is highly reactive, it can be dangerous when mishandled, causing damages to the laboratory and injury or even death to people or animals in the vicinity.
Methyl iodide is a methanol and hydrogen iodide byproduct when acetic acid is prepared through the Monsanto process. The methyl iodide reacts with carbon monoxide in this process when a rhodium complex is present and produces acetyl iodide, which can then be further refined.
Methyl Iodide as a Pesticide
Methyl iodide’s toxic properties make it useful as a pesticide. Insects, nematodes, fungi, and specific herbs can be controlled with methyl iodide. It can be used as a spray or can be injected into the ground. Although methyl iodide is good as a pesticide, its toxicity to humans and animals such as livestock make its use very controversial.
Methyl iodide has been approved to be used as a pesticide. It is a fungicide, herbicide, insecticide, nematicide, and soil disinfectant. It is commonly used in the US, Mexico, Morocco, Japan, Turkey, and New Zealand. The registration of methyl iodide as a pesticide is pending in many other countries. Although proven toxic, methyl iodide is still used in several locations.
Toxicity of Methyl Iodide and Biological Effects
Methyl iodide has been classified as a chemical that can cause/accelerate cancer and is toxic to the reproductive system. Excessive iodide intake impairs thyroid function in humans and becomes a carcinogen.
Methyl iodide has a median lethal dose of 76 mg/kg in rats. The liver rapidly converts methyl iodide into S-methylglutathione. Depending on the length of exposure and amount absorbed into the system, methyl iodide fumes can damage the lungs, liver, kidneys, and central nervous system. Nausea, dizziness, coughing, and vomiting are common symptoms of such exposure and long term exposure can cause skin burns. A pulmonary edema can result from massive inhalation of the fumes. Overexposure to the fumes or liquid forms of methyl iodide can result in death. Such exposure usually occurs when someone is in the vicinity when a methyl iodide leak or accidental over exposure occurs.
Exposure to farmland and water contamination can also cause health concerns for humans and animals. Pregnant women who are exposed to methyl iodide can lose the fetus at any stage of development due to the toxicity of the compound. Women who are exposed to large amounts of methyl iodide can also become very ill or expire because a pregnancy is taxing on the body or when the body tries to relieve itself of the toxic chemical.
Why Farmers Continue to Use Methyl Iodide
Methyl Iodide is an effective insecticide and is relatively cheap. Its residual effects can last longer than a single season in many locations and can help to reduce the cost of keeping crops treated, especially where the growth season is very short for some types of crops.
As a fumigant, methyl iodide reduces the number of mice, moles, rats, and other pests that could significantly damage crops when not controlled. Responsible farmers who use methyl iodide to control pests can control its toxicity. Methyl iodide use must be precise to really ensure that its toxicity is kept at a harmless level.
Methyl iodide can be used on crops such as berries, fruits, tubers, and root vegetables. More than 100 crops benefit from the chemical as it controls the pests that commonly prey on them, resulting in larger yields throughout the season. Since methyl iodide controls weeds and other problematic plants, less manpower is required to maximize production.



Follow Us!