There are many different kinds of energy but the single most widely used in society as well as in nature is chemical energy. Chemical energy allows energy to be stored for later use and it is also easy to access. Without chemical energy, many of the luxuries and even basic necessities that people have grown accustomed to would not be possible such as vehicular transportation, food, clean air, and battery-operated devices. This article will explain what chemical energy is and how it is used in nature and society.
What is Chemical Energy
Chemical energy is energy that can be stored and broken down by reactions with other elements and chemicals. Chemical energy is derived from the bonds that connect atoms with other atoms which release energy when they are created and require energy in order to break them. Chemical energy is what allows plants to convert carbon dioxide into oxygen, food to be digested and even exist in the first place, and combustion engines to work. The following are some examples of how chemical energy plays an important role in both society and the environment.
Batteries
Batteries work by using multiple chemicals that interact with one another to create electrical energy. The most widely used type of battery is a zinc-copper battery which uses the chemicals zinc and copper, zinc being the negative side and copper being the positive side. When a wire is a connected to both ends, the acid inside the battery is excited and breaks down some of the zinc. The electrons from that zinc are then free to travel along the wire to the copper side where electricity is conducted and then able to flow into the device that the battery is connected to.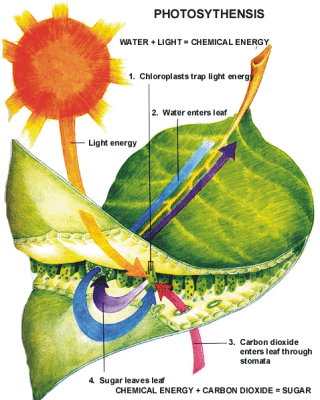
Photosynthesis
Photosynthesis is the process in which plants break down carbon dioxide and water and convert them into oxygen and sugar. Photosynthesis requires that the plant absorbs sunlight, water, and carbon dioxide. The sunlight causes oxygen atoms to leave the cells inside the plant while the carbon and hydrogen are used to create sugars for the plant to store and consume. Without this process, plants could not survive and neither could the animals that eat the plants nor the animals that eat those animals.
Food
Food is the most basic necessity to life and every creature requires some form of it. Food, however, is a form of chemical energy. When plants create sugar through photosynthesis, they provide animals such as bees, rabbits, birds, and insects with a steady food source. Those creatures use some of the energy that they absorbed to function while the rest is stored for later use. By storing extra energy, however, they provide a steady food source for other animals such as foxes, wolves, dogs, and cats. Likewise, those animals store some of the energy they absorb and are eventually eaten by even larger animals such as bears, lions, and humans. The entire food chain is based on chemical energy.
Gasoline
Gasoline is the basis for vehicular transportation and works by releasing a tremendous amount of energy compared to the amount that is actually used. In an internal combustion engine, a valve opens up to allow a tiny drop of gasoline and a lot of air into a cylinder. The incoming air causes a device known as a piston to be pushed down until the entire cylinder is full of air. The piston is connected to a device called a crankshaft which rotates and pushes the piston back up when the cylinder is full. As the piston is pushed back up, the gasoline and air is compressed into a dense gas. When the piston reaches the top of the cylinder, a spark plug ignites the gas. The energy that is released is then directed, focused, and sometimes converted into electrical energy by other devices. The energy is used to move the vehicle as well as power any electrical devices that the car supports. While these man-made devices do a lot of the work, none of it would be possible without the chemical energy found within the gasoline.



Follow Us!