Isomers are compounds with the same molecular, but different structural formulas. Isobutane is an isomer of butane. It belongs to a class of compounds called alkanes, which are chains of carbon atoms where each carbon atom is attached to as many hydrogen atoms as possible. Its chemical formula is C4H10. Butane consists of four carbon atoms. With four such atoms, it is possible to link the atoms in two completely different ways. The first way, where all the four atoms are linked in a row, is referred to as Butane. The other way, where a carbon atom in the middle links the other three carbon atoms and a hydrogen atom, is known as Isobutane.
Nomenclature
Isobutane is actually not a butane, as its longest chain of carbon atoms has only three atoms. It is for this reason that it has been given the name Methylpropane. It is not necessary to mention this name with the position number (2-methylpropane), as this is the only possible position in methylpropane. As a result, the International Union of Pure and Applied Chemistry has retained Isobutane as its trivial name.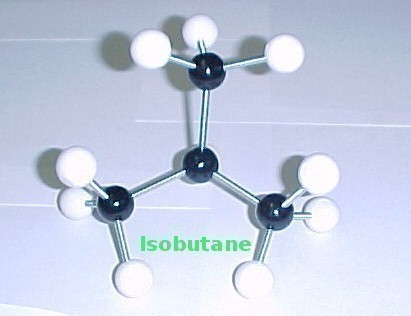
Isobutane Uses
Aerosols
Isobutane is widely used in aerosol spray production. Adding isobutane to a wide variety of products’ formulas, ranging from cooking spray to hair spray, adds the required propellant quality. That is to say, the product can easily be expelled from its container, doing away with the need for a hand pump sprayer.
Refrigeration
Freon and other similar substances are mainly used in compounds that aid the refrigeration process. In recent years however, serious concerns have been raised about the environmental viability of these substances, as their emissions have been linked to ozone layer damage. Fortunately, isobutane, which has none of the destructive properties of Freon and related compounds came along. As a result, it is widely used in all kinds of refrigeration systems today, ranging from small home solutions to huge commercial freezers. When used as a refrigerant or as a propellant in aerosols, isobutane is referred to as R-600a.
Petrochemical Industry
Isobutane’s most important use in the petrochemical industry is its role as a feedstock that aids in the creation of the perfect environment for isooctane production. There is ongoing research on isobutane’s capabilities as a feedstock in enhancing the production of various other compounds.
Air Conditioning
With isobutane making giant strides in the refrigeration field, its use in the air conditioning industry is not far behind. There are plans on the drawing board for it to be used in domestic air conditioners, large central cooling systems, and vehicle cooling systems.
Safety Issues
There were several reports from the UK in 2009 about isobutane leaking into refrigerator cabinets and sparks from the electrical system igniting the leaked isobutane, thereby causing explosions. Though these are unconfirmed reports, isobutane is a flammable gas, and its use is not without risk. Chlorofluorocarbons (CFCs/trade name – Freon) that were previously used in refrigerators depleted breathable air and lead to frosting at the escape area. Isobutane poses the additional risk of explosion.
According to its MSDS (Material Safety Data Sheet) information, Isobutane should not be exposed to temperatures above 52 degrees Celsius or 151 degrees Fahrenheit when it is kept in a closed environment such as a refrigerator. The flammable range for isobutane is 1.8% – 8.4%. This means that if there is a leak in the refrigeration system, any nearby ignition sources can trigger an explosion and cause damage to people and their property. Such leaks should be immediately reported and checked in open air to ensure the technician’s safety. In most cases, a leak renders the refrigeration system useless, calling for a full replacement.
Why is Isobutane Safer for the Environment?
Isobutane (or R-600a) was traditionally used as fuel for lighters and stoves. Since traditional aerosols and refrigerants’ negative environmental impacts were discovered, isobutane has replaced them. Chlorofluorocarbon refrigerants such as R-12, R-22, and R-134a are examples of substances that negatively impact the environment. Isobutane, on the other hand, has negligible ozone depletion and Global Warming Potential. It is also a feedstock for creating environments for isoctane production and for enhancing other industrial compounds that are not ozone friendly.


Follow Us!