FTIR spectroscopy (Fourier Transform Infrared Spectroscopy) is a technique that uses infrared light to observe properties of a solid, liquid, or gas. It is used in many different applications to measure the absorption, emission, and photo-conductivity of matter by shining a narrow beam of infrared light at the matter in various wavelengths and detecting how the matter responds to each wavelength. Once the data has been obtained, it is converted into digital information using a mathematical algorithm known as the “Fourier transform.”
How FTIR Spectrocopy Works
FTIR spectroscopy is closely related to dispersive spectroscopy, but the actual technique used is different. In dispersive spectroscopy, a monochromatic beam of light is shone at a sample in different wavelengths and the absorption of light is measured each time. In FTIR spectroscopy, a beam of light is passed through a series of mirrors that cause the beam’s individual wavelengths to hit each other in a way that allows a sample to absorb some wavelengths, while others are blocked. Light absorption is measured and a computer infers the absorption rate of each wavelength within the beam.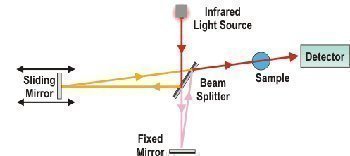
Applications
FTIR spectroscopy can be used to separate individual molecules in gases in order to detect isomers and other compounds, as well as analyze the properties of the gas. FTIR spectroscopy can also be used to detect emissions from a sample, such as in the case of luminescence, radioactivity, and thermal applications.
Advantages
FTIR spectroscopy can be performed much faster than dispersive spectroscopy, allowing it be used in much more diverse applications. This is especially true when measuring gases, which move very quickly and may therefore obscure results. This is because FTIR spectroscopy measures the absorption rate of many different frequencies simultaneously, a property that has led it to make dispersive spectroscopy obsolete.
Disadvantages
Because FTIR spectroscopy measures the absorption rate of a beam of light as a whole, it must infer the individual absorption rates of each wavelength of light. This means that FTIR spectroscopy cannot be completely accurate about the absorption rate of any wavelength within the beam. In contrast, dispersive spectroscopy measures each wavelength individually and is completely accurate.




Follow Us!