Potassium benzoate is the potassium salt of benzoic acid. Benzoic acids are weak acids that are used as food preservatives. Potassium benzoate is also commonly used for preserving food.
It is primarily used to inhibit mold and bacteria growth. This allows the product that potassium benzoate preserves to last longer on the shelf, thereby increasing profits for the company that produced it. The ideal pH for products using potassium benzoate as a preservative is below 4.5 because that is where it exists as benzoic acid.
Acidic food or beverages such as fruit juice, pickles, and soft drinks can be preserved with potassium benzoate. Most countries including Canada, the U.S., and the EU approve it. However, the EU is against children consuming products that are preserved with it. Europeans use an E number to categorize food additives, potassium benzoate’s is E212.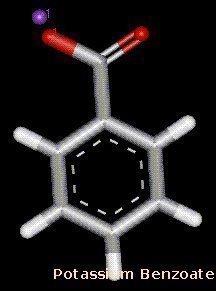
Potassium benzoate is also used to produce the whistle in many fireworks.
Making Potassium Benzoate
There are a few ways to make potassium benzoate. The first and main way of making it is to oxidize toluene. Oxidization simply means that one electron (or more) has been removed.
Another way to do it in the lab is to react methyl benzoate with potassium thioacetate. The methyl attaches to the thioacetate and the potassium attaches to the benzoate when this reaction occurs, which results in methyl thioacetate and potassium benzoate.
The Dangers of Potassium Benzoate
Potassium benzoate (on its own) is not very dangerous so long as the bottles that hold the drink are kept in proper and safe conditions. However, this never happens when shipping drinks as the bottles get hot and more light gets into the shipping vessel.
When potassium benzoate and ascorbic acid (vitamin C) are mixed, and heat and light are added to it, benzene can be formed. Benzene is a known carcinogen. The American Petroleum Institute stated in 1948 that, “it is generally considered that the only absolutely safe concentration for benzene is zero.” Because of that, it is important that drinks with potassium benzoate are properly shipped.
The Food Commission in the UK described potassium benzoate as, “mildly irritatant to the skin, eyes, and mucous membranes.”


Follow Us!